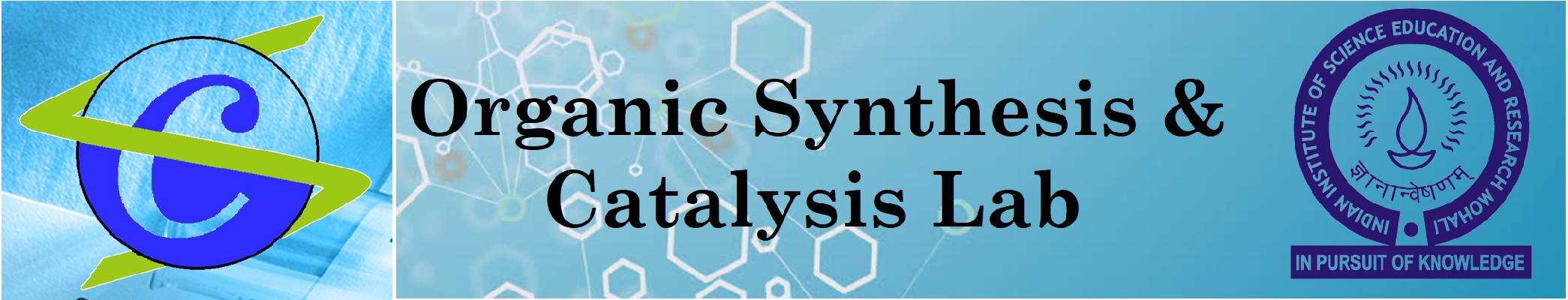
Our research philosophy is that the methods we develop should be user-friendly, experimentally trivial, environmentally friendly, and economically sound while providing access to otherwise difficult targets of structural and biological significance.
ii) Development of green and sustainable synthetic chemistry and atom economic reactions.
iii) Application of aforementioned strategies in the total synthesis of bioactive natural products and pharmaceutically important compounds.
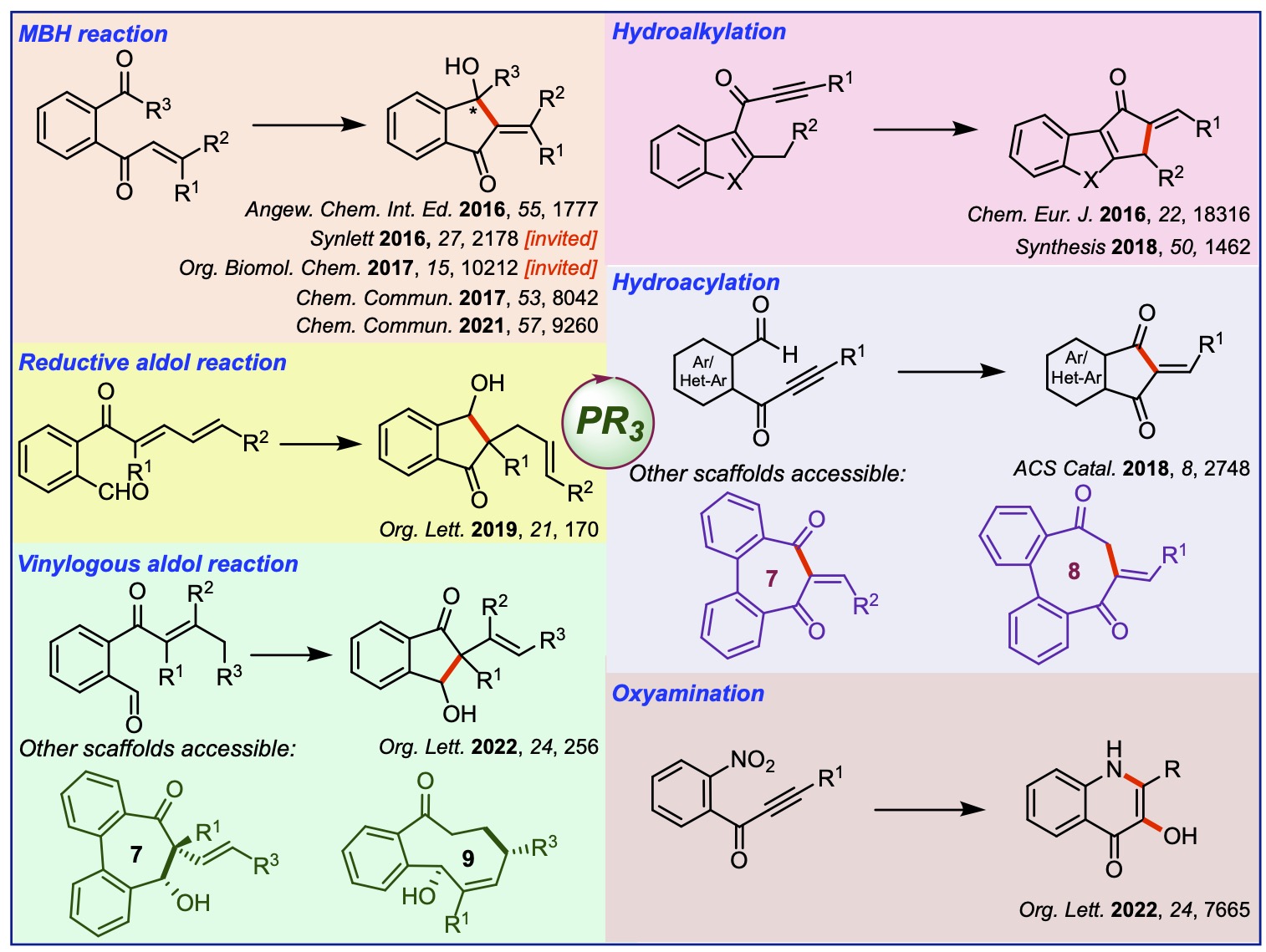
Our group actively works on developing novel Pd-catalyzed cycloisomerization reactions. We have showcased the utility of these methods in synthesizing many bioactive natural products. We have also assembled several intricate scaffolds through these strategies, which have relevance in medicinal chemistry and materials science. Some of the key contributions to this area are summarized below.
Accounts and reviews in this area:
III. New chemistry with sulphur ylides
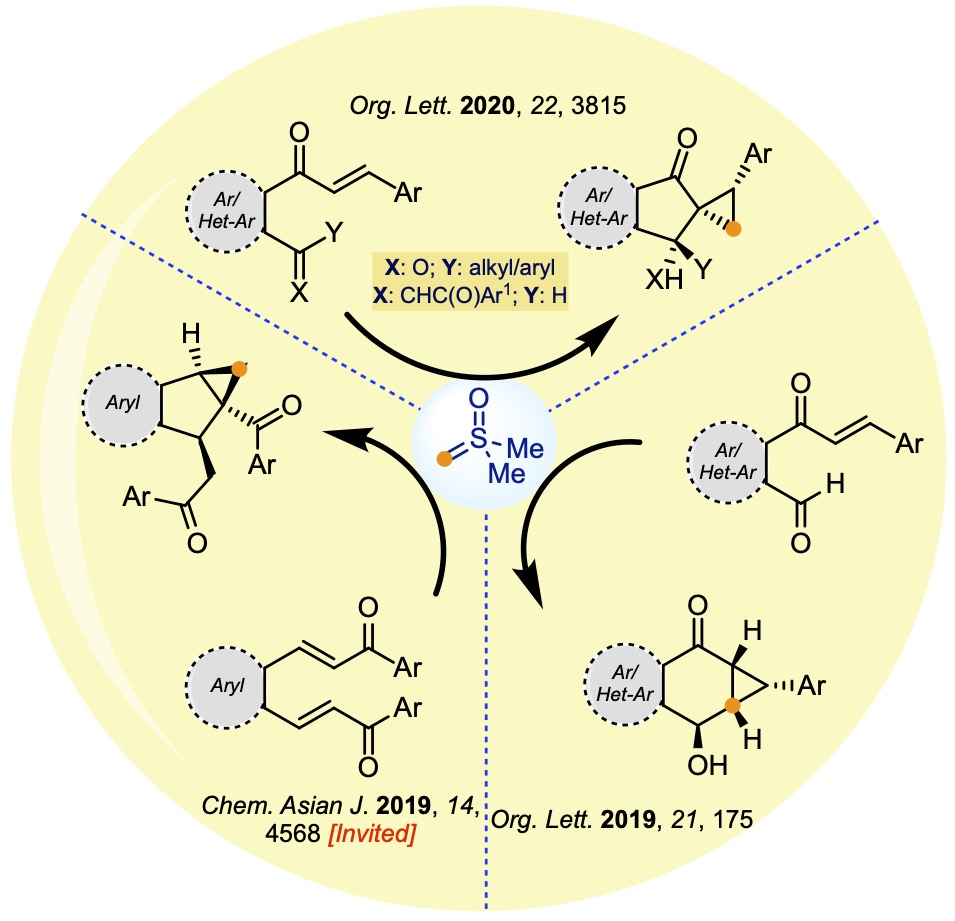
IV. One-pot cascade transformations with Lewis and Brønsted acids/basesSulfur ylides are traditionally employed to synthesize cyclopropanes, epoxides, and aziridines from the respective olefins, carbonyls, and imines. We made a serendipitous entry into this field and articulated several one-pot cascade transformations employing sulfur ylides as reagents that provided facile access to unprecedented cyclopropanoids. We have manipulated the complexity of the molecular scaffolds by the rational design of the substrates. We have summarized some of the recent developments in the following figure.

| Metal-catalyzed one-pot cascades | Metal-free one-pot cascades |
|
|